Overview
| A lowest priced hypertonic sodium chloride solution for inhalation in NHS |
| Up to 58% cost savings |
| Listed in Drug Tariff |
| Preservative Free |
| Bio-equivalence to leading Brands |
| Available from mainline wholesalers – High level of stocking |
PulmoClear 3%, 6%, 7% Increases the mobilisation of secretions in the lower respiratory tract by osmotic effects and prevents drying of bronchial mucous. This hypertonic saline solution for inhalation can be used with pneumatic or ultrasonic nebulisers.
| A lowest priced hypertonic sodium chloride solution for inhalation in NHS |
| Up to 58% cost savings |
| Listed in Drug Tariff |
| Preservative Free |
| Bio-equivalence to leading Brands |
| Available from mainline wholesalers – High level of stocking |

| A lowest priced hypertonic sodium chloride solution for inhalation in NHS |
| Up to 30% cost savings |
| Listed in Drug Tariff |
| Preservative Free |
| Bio-equivalence to leading Brands |
| Available from mainline wholesalers – High level of stocking |
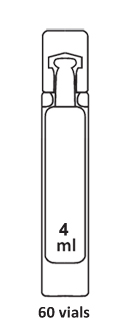
PULMOCLEAR 3% : Pack Size – 60 vials X 4ml
PULMOCLEAR 6% : Pack Size – 60 vials X 4ml
PULMOCLEAR 7% : Pack Size – 60 vials X 4ml
Sodium Chloride: 3g / 6g / 7g, Purified water q.s.100 mL, Preservative free

For Inhalation Use Only. | |
The recommended dose of Hypertonic saline solution for inhalation is one unit-dose vial 2 to 4 times a day, undiluted via a nebuliser. | |
PulmcoClear strength and dose should be individualised based on the status of the patient, age group and should be assessed at regular intervals of symptoms. |
Prescribing PulmoClear by brand is the least expensive way of prescribing 3%, 6% and 7% Hypertonic Sodium Chloride Inhalation solution. | |
PulmoClear costs approximately 30% less than Nebusal & MucoClear, or prescribing Generic Hypertonic Sodium Chloride Inhalation solution. | |
Every Year NHS spends approximately more than £ 2m on hypertonic saline solution for inhalation. By switching to PulmoClear, NHS can save upto £ 500,000 a year. |
|
Product |
Strength |
Pack Size |
NHS Price |
NHS Cost Saving |
PIL |
|---|---|---|---|---|---|
|
PulmoClear 3% |
60 X 4ml |
£16.47 |
----- |
PIL | |
|
MucoClear 3% |
60 X 4ml |
£38.94 |
58% |
||
|
Resp-Ease 3% |
60 X 4ml |
£21.60 |
24% |
||
|
PulmoClear 6% |
60 X 4ml |
£16.59 |
----- |
PIL | |
|
MucoClear 6% |
60 X 4ml |
£38.94 |
57% |
||
|
Resp-Ease 6% |
60 X 4ml |
£21.60 |
23% |
||
|
PulmoClear 7% |
60 X 4ml |
£16.59 |
----- |
PIL | |
|
Nebusal 7% |
60 X 4ml |
£27.00 |
38% |
||
|
Resp-Ease 7% |
60 X 4ml |
£21.60 |
23% |
In cases of cystic fibrosis, bronchiectasis, bronchiolitis; PulmoClear increases the mobilisation of secretions in the lower respiratory tract by osmotic effects. | |
PulmoClear prevents drying of bronchial mucous. | |
PulmoClear hypertonic saline solution for inhalation can be used with pneumatic or ultrasonic nebulisers. |
For Inhalation Use Only. | |
The recommended dose of Hypertonic saline solution for inhalation is one unit-dose vial 2 to 4 times a day, undiluted via a nebuliser. | |
PulmcoClear strength and dose should be individualised based on the status of the patient, age group and should be assessed at regular intervals of symptoms. |
|
Product |
Strength |
Pack Size |
NHS Price |
NHS Cost Saving |
PIL |
|---|---|---|---|---|---|
|
PulmoClear 3% |
60 X 4ml |
£18.94 |
----- |
PIL | |
|
MucoClear 3% |
60 X 4ml |
£27.00 |
30% |
||
|
Resp-Ease 3% |
60 X 4ml |
£21.60 |
12% |
||
|
PulmoClear 6% |
60 X 4ml |
£18.94 |
----- |
PIL | |
|
MucoClear 6% |
60 X 4ml |
£27.00 |
30% |
||
|
Resp-Ease 6% |
60 X 4ml |
£21.60 |
12% |
||
|
PulmoClear 7% |
60 X 4ml |
£18.94 |
----- |
PIL | |
|
Nebusal 7% |
60 X 4ml |
£27.00 |
30% |
||
|
Resp-Ease 7% |
60 X 4ml |
£21.60 |
12% |
TriOn Pharma are aware that when Health professionals, CCGs and Health Boards consider a branded medicine/device, continuity of supply and price are key aspects of your due diligence. To reassure you and support your due diligence we are happy to make commitments on the price and supply of PulmoClear.
Please contact us at info@trionpharma.co.uk for a bespoke assurance letter for your CCG or Health Board.
We recognise the importance of continued supply of branded medicines/devices and are committed to the highest quality standards, ensuring stock is available to the NHS across all parts of the UK. Our experienced team have a proven track record of over 15 years supplying medicines and devices to both primary and secondary care organisations across the UK.
We are therefore committed to ensuring sustainable supply. For further information about our commitment, please contact us at info@trionpharma.co.uk
PulmoClear has identical indications and Bioequivalence to leading brands like Nebusal & Mucoclear.
PulmoClear has similar composition/formulation to leading brands.
Cystic Fibrosis (CF): Delivered following a bronchodilator, hypertonicsodium chloride 7% appears inexpensive and safe with no increasedinfection risk. A Cochrane review stated that there is sufficientevidence to recommend using hypertonic sodium chloride 7% inthose with CF (Wark 2009) (Elkins 2006). | |
It was found that Hypertonic Saline inhaled twice per day reduced episodes of chest infection and was linked to lung function andquality of life improvement, and better attendance at school or work. | |
Bronchiectasis: British Thoracic Society Guidelines for the treatment of non-CF bronchiectasis suggest that hypertonic sodium chloridemay be considered for use prior to airway clearance to increasesputum yield, reduce sputum viscosity and improve ease of expectoration (BTS July 2010). | |
Nebulized HS is a safe and potentially effective treatment of infantswith acute bronchiolitis. | |
In concentrations of 3-14%, hypertonic saline has been shown toimprove tracheobronchial clearance in patients with chronicbronchitis, CF, asthma and normal individuals. | |
Hypertonic saline resulted in an improvement in measures of mucociliary clearance over isotonic saline and cough alone (Riedler 1996; Robinson 1996; Robinson 1997; Robinson 1999). |
Hypersensitivity to inhalation Sodium Chloride solution. | |
The solution should not be administered orally or parenterally. | |
Do not mix with other medications. | |
Single use only. | |
Strictly reserved to inhalation. Not for injection. | |
Follow the nebuliser manufacturer's instructions for use. |
No known interactions. All strengths of PulmoClear can be effectively and safely used in conjunction with standard therapies such as antibiotics, bronchodilators, pancreatic enzymes, vitamins, inhaled and systemic corticosteroids and analgesics.
In rare cases, temporary irritations (e.g. coughing) or reversible constriction of the bronchia can occur.
No known interactions. All strengths of PulmoClear can be effectively and safely used in conjunction with standard therapies such as antibiotics, bronchodilators, pancreatic enzymes, vitamins, inhaled and systemic corticosteroids and analgesics.
In rare cases, temporary irritations (e.g. coughing) or reversible constriction of the bronchia can occur.